क्रिस्टल जालक और एकक कोष्ठिका ( CRYSTAL LATTICE AND UNIT CELL )
क्रिस्टल जालक - किसी क्रिस्टलीय ठोस की आंतरिक संरचना में अणु , परमाणु या आयन एक विशेष व्यवस्था द्वारा बिन्दुओं के रूप में जमे रहते हैं । त्रिविम आकाश में अणु , परमाणु अथवा आयनों की इस नियमित ज्यामितीय व्यवस्था को ही क्रिस्टल जालक कहते हैं । यह उच्च अनुक्रमिक संरचना है जो कि अवयवी या रचक कणों की प्रकृति के कारण क्रमबद्ध रूप में बनती है ।
क्रिस्टल जालक के अभिलक्षण
1. किसी क्रिस्टल में अवयवी कणों ( परमाणु , अणु या आयन )की त्रिविमीय व्यवस्था में प्रत्येक कण को एक बिन्दु द्वारा दर्शाया जाता है । इसे जालक बिन्दु ( Lattice point ) कहते है ।
2. क्रिस्टल जालक में प्रत्येक जालक बिन्दु एक अवयवी कण ( परमाणु , अणु अथवा कण ) को दर्शाता है ।
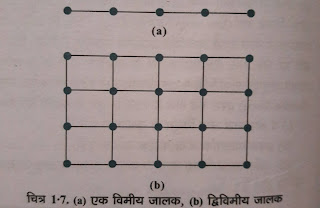
3. जालक बिन्दुओं को सीधी रेखाओं द्वारा जोड़ा जाता है जिससे कि जालक की ज्यामिति को व्यक्त किया जा सके । हॉय के अनुसार , जब अवयवी कणों की पुनरावृत्ति एक सरल रेखा में होती है , तो इसे एकविमीय जालक ( one dimensional ) किसी तल में होने पर द्विविमीय जालक ( Two - dimensional ) ( चित्र 1-8a ) और कणों की पुनरावृत्ति त्रिविम में होने पर त्रिविमीय जालक ( space lattice ) ( चित्र 1-8b ) कहते हैं ।
चित्र 1-7 . ( a ) एक विमीय जालक , ( b ) द्विविमीय जालक
एकक कोशिका या एकक सेल ( Unit Cell ) - किसी क्रिस्टल जालक का वह सूक्ष्मतम भाग ( बहुत छोटी - छोटी समान इकाइयों ) , जिसकी त्रिविम में बारम्बार पुनरावृत्ति करने पर संपूर्ण क्रिस्टल जालक का निर्माण हो जाता है , उसे एकक कोष्ठिका या इकाई सेल कहते हैं ।

चित्र 1.8 . ( a ) इकाई सेल एवं ( b ) त्रिविमीय जालक
एकक कोष्ठिका के प्राचल ( Parameters of Unit Cells ) एकक कोष्ठिका को परिभाषित करने वाले पैरामीटर निम्नलिखित हैं
( i ) एकक कोष्ठिका के तीनों किनारों की विमाओं ( अक्षों की लंबाई ) को a , b और c द्वारा दर्शाते हैं , जो परस्पर लंबवत् हो भी सकते है , अथवा नहीं भी ।
( ii ) किनारों के मध्य कोण ८ , B और द्वारा दर्शाए जाते है इस प्रकार एकक कोष्ठिका इन्हीं 6 पैरा ... c , B और ) द्वारा अभिलक्षित होती हैं ?
एकक या इकाई सेल के प्रकार ( Types of Unit Cells ) इकाई सेल मुख्यतः दो प्रकार के होते हैं
1. आद्य या सरल घनीय ( Primitive or simple unit cell ) इस प्रकार के इकाई सेल में जालक बिन्दु केवल कोनों पर उपस्थित होते हैं ।
2. केन्द्रित एकक कोष्ठिका ( Centred unit cell ) - जब एकक कोष्ठिका में एक अथवा अधिक अवयवी कण कोनों के अतिरिक्त अन्य स्थितियों पर भी उपस्थित होते हैं , तो उसे केन्द्रित एकक कोष्ठिका कहते हैं । यह तीन प्रकार की होती है
( a ) फलक केन्द्रित ( Face - centred ) - इस प्रकार के यूनिट सेल में जालक बिन्दु सभी कोनों के साथ - साथ प्रत्येक फलक के मध्य बिन्दु पर भी स्थित होते हैं । जैसे- NaCl .
( b ) अन्तः केन्द्रित या काय केन्द्रित ( Body - centred ) - इस प्रकार के यूनिट सेल में जालक बिन्दु सभी कोनों के साथ - साथ पूरे यूनिट सेल के मध्य में भी होता है । जैसे- Cscl .
( c ) अंत्य केन्द्रित ( End - centred ) - इस प्रकार के यूनिट सेल में जालक बिन्दु सभी कोनों में तथा किन्हीं दो विपरीत फलकों के केन्द्र पर भी स्थित होते हैं ।